IN DEPTH: EBOLA OUTBREAK IN THE DEMOCRATIC REPUBLIC OF CONGO
Public health emergency of international concern
It has been one year since an Ebola virus disease (EVD) outbreak has been declared in the Democratic Republic of Congo (DRC). To date, there have been nearly 3,000 human cases with over 1,800 deaths in the provinces of North Kivu and Ituri. While three cases of EVD have been identified in Uganda in July, disease transmission was suspected to have occurred in the DRC. In the same month, a case was confirmed in Goma, the capital of North Kivu with a human population of 2 million.
The case in Goma is significant because this provincial capital is a major hub for international airline travel. Now, with an increased risk for global EVD spread - in addition to other challenges hampering disease containment such as deadly violence to health care workers, weak disease surveillance and public health infrastructure, and public mistrust - the World Health Organization (WHO) declared this outbreak a “Public Health Emergency of International Concern (PHEIC).” This declaration, by definition, classifies the EVD situation in the DRC “an extraordinary event that poses a public health risk to other countries through international spread and that potentially requires a coordinated international response.”
As a result of this declaration, the World Bank is mobilizing $300 million to strengthen preparedness, health systems, and response. Increased funding has led to an estimation of 3-5-fold increase in patients seeking access to healthcare, the creation of 10 EVD treatment centers, the addition of over 5,00 handwashing stations, hazard pay for over 60% of healthcare workers, and the vaccination against EVD of over 80,000 people among other resources with the ultimate goal of decreasing EVD spread.
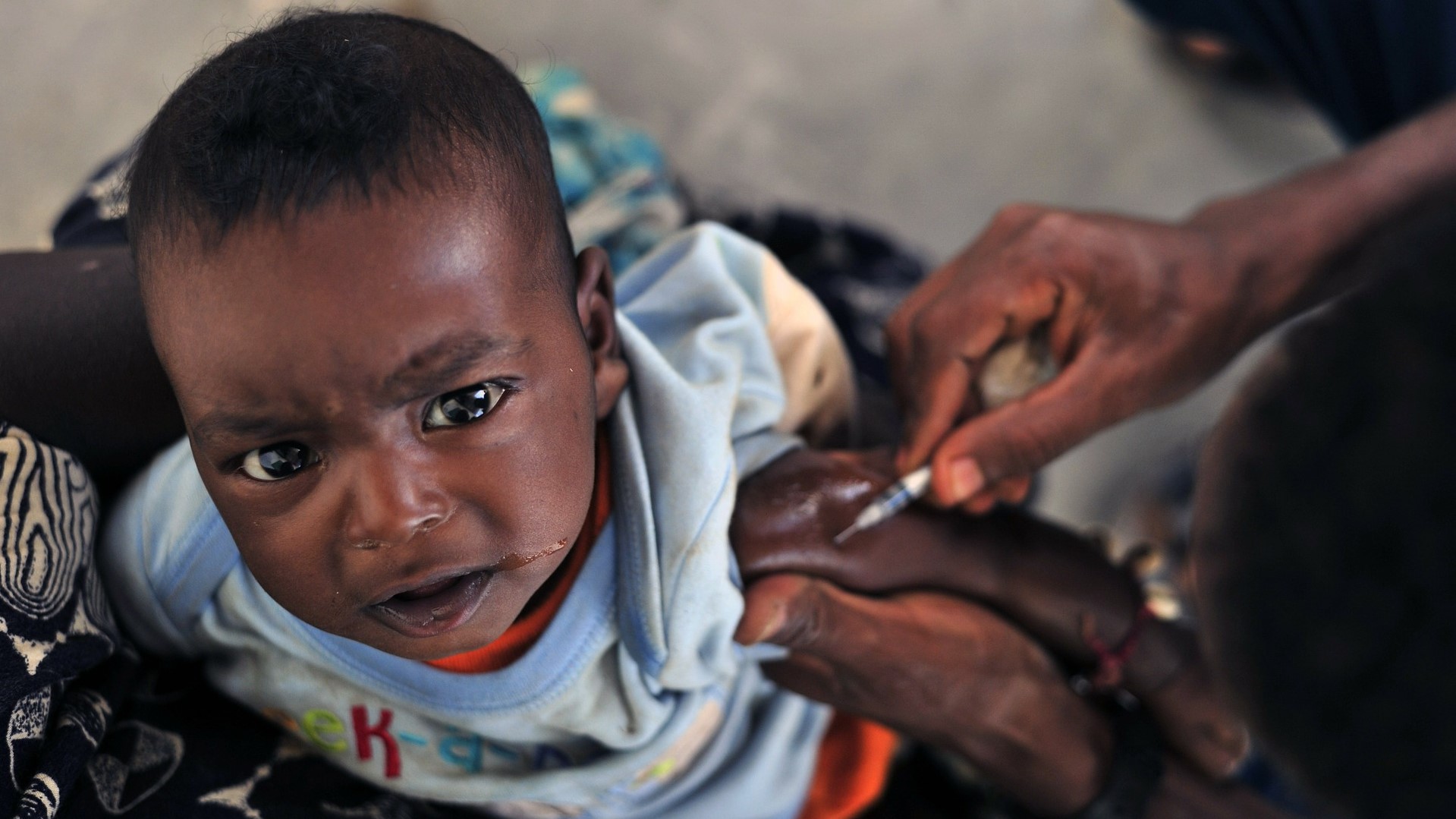
Ebola Vaccine
Currently, there is no licensed vaccine against the deadly EVD. However, while unlicensed, the rVSV-ZEBOV vaccine is currently being used on a “compassionate use” (or “expanded access”) in the DRC outbreak. This vaccine, produced by Merck (MSD), has been shown to be safe and effective against the Zaire strain of the Ebola virus and is being disseminated in “ring vaccination” methodology. An unlicensed new vaccine (or drug) may be used on a “compassionate use” basis in situations where there are no other therapy options outside a completed clinical trial - with the authorization of the affected government. The final stages of clinical trials have not yet been completed for this vaccine.
This vaccine, rVSV-ZEBOV, is a single dose live-attenuated vector based virus vaccine. The virus base of this vaccine is Vesicular Stomatitis Virus (VSV) which can cause transient disease - vesicles on hoof and mouth - in cattle, swine, horses and can cause mild or transient illness in humans. Side effects of this vaccine, if they occur, are mild causing headache, fatigue, muscle pain, and fever. Vaccine recipients are still advised to avoid potentially infected EVD bodily fluids and materials which may have been contaminated.
The ring vaccination strategy relies on recruiting at risk individuals voluntarily, who were in close proximity to a laboratory confirmed EVD patients within the last 21 days. This includes neighbors, family members, and healthcare workers in contact with EVD patients. An average of 150 persons may be vaccinated per each identified case. In reality, about 50 people are being vaccinated per identified case.
New vaccine trial proposed
While the currently used vaccine strategies have been shown to be safe and effective, The WHO’s Emergency Committee for Ebola virus disease have concluded vaccine supplies are currently insufficient to control the current outbreak if it persists. The current Ebola response coordinator in Congo, Jean-Jacques Muyembeour, predicts the current outbreak could last 2-3 more years. Experts have suggested the need to widen the ring of contacts, vaccinating more people. Merck has confirmed there is enough vaccine to immunize nearly 500,000 people at the current dose, and plans to double the supply in 2020. There are approximately 10 million people in the affected provinces. Proposed solutions include reducing the administered dose and deploying a second vaccine.
The new vaccine (Ad26.ZEBOV and MVA-BN-filo) is manufactured by Janssen, the vaccine division of Johnson and Johnson, and is also unlicensed. This vaccine is administered in two doses and was initially created to prophylactically protect healthcare workers from EVD. In the DRC, the vaccine would be administered to those individuals who are further removed, outside of the rings of contacts from an EVD confirmed case: vaccines would be administered in areas outside the active outbreak zone. The goal is to build up more immunity in the community, building a “protective wall.” This proposed trial would be carried out by Ugandan researchers in the DRC - with involvement of Congolese scientists - and would include 800 people with a proposed timeline of two years.
The DRC government has expressed concerns regarding this new vaccine: public mistrust with administering two separate vaccines and the logistical practicability of administering two doses, two months apart. Dr Oly Ilunga, the former health minister for the DRC said, “We are in a very critical outbreak in a very complex environment,” Ilunga said. “We want to have all the human resources dedicated to the outbreak. We don’t want people to be diverted in another clinical trial elsewhere in the country.” This proposed vaccine trial is currently still in debate.
